Patient Registry Leads Researchers to Discover Two More Genetic Defects Associated with Congenital Dyserythropoietic Anemias
Theodosia Kalfa, MD, PhD, established the Congenital Dyserythropoietic Anemias Registry (CDAR) five years ago. Now, the registry is helping to advance understanding of CDA, its genetic causes and pathways that are essential for normal erythropoiesis.
Through their work with the registry, Kalfa and her colleagues have found two previously unrecognized causative genetic defects and are exploring several others. These defects were discovered in the VPS4A and PRDX2 genes.
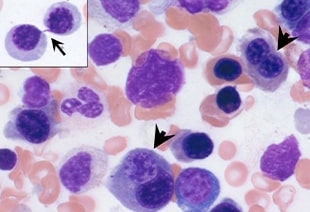
CDA is characterized by chronic anemia, iron overload and the distinctive finding of multinucleated erythroblasts in the bone marrow. Researchers have identified five major types (CDA 1-5), but up to half of all cases cannot be classified.
“CDAs are poorly understood, difficult to diagnose and challenging to treat,” says Kalfa, a pediatric hematologist at Cincinnati Children’s. “A pediatric hematologist might go years without encountering a new diagnosis. Our ultimate goal is to find treatment targets and develop novel therapies not only for CDA but also for common hematologic diseases characterized by ineffective erythropoiesis and iron overload.”
CDAR, the first registry of its kind in North America, includes a database and biorepository for CDA. As of September 2021, the registry had enrolled 107 individuals (55 patients and 52 family members) from 34 institutions.
Discovery of the VSP4A Gene’s Role

Many of the patients enrolled in the registry learned about it after receiving results from a gene panel available at Cincinnati Children’s. About 55% of the patients enrolled with clinical and pathologic diagnosis of CDA do not have one of the known genetic defects associated with the disease.
Three patients enrolled in the registry had been diagnosed with CDA and severe neurodevelopmental delay. Initial testing on a CDA-gene panel did not reveal a causative mutation, indicating a non-typeable CDA, but whole-exome sequencing pointed to a mutation in the VSP4A gene. The report of this discovery was published in The American Journal of Human Genetics, 2020.
In 2020, Kalfa and fellow researchers in CDAR also published on the natural history of CDA-I (Blood Cells, Molecules and Diseases, December 20, 2020).
The registry has become a focal point for hematologists who want to contribute to the scientific understanding of this rare disease. Recently researchers from the University of Michigan-Ann Arbor began accessing the biorepository for their CDA-II research.
Patients of all ages can request CDA genetic testing at Cincinnati Children’s and enroll in the CDA registry. To learn more, contact Theodosia.Kalfa@cchmc.org.

